Abstract
The goal of our study was to create a nomogram to predict the risk of developing hypertension in patients with periodontitis. Our study used data from a total of 3196 subjects from the National Health and Nutrition Examination Survey 2009 to 2014 who had ever been diagnosed with periodontitis. The data set was randomly divided into a training set and a validation set according to a 7:3 ratio. The data from the training set was utilized to build the prediction model, while the validation set were used to validate the model. To identify the risk variables, stepwise regression was used to perform successive univariate and multivariate logistic regression analysis. The predictive ability of the nomogram model was evaluated using receiver operating characteristic curve. Calibration plots were used to assess the consistency of the prediction model. The clinical value of the model was evaluated using decision curve analysis and clinical impact curve. A nomogram for the risk of hypertension in subjects with periodontitis was constructed in accordance with the 8 predictors identified in this study. The areas under the receiver operating characteristic curve values for the training set and validation set were 0.922 (95% confidence interval: 0.911–0.933) and 0.918 (95% confidence interval: 0.900–0.935), respectively, indicating excellent discrimination. The decision curve analysis and clinical impact curve suggested that the model has significant clinical applications, and the calibration plots of the training set and validation set demonstrated good consistency. The nomogram can effectively predict the risk of hypertension in patients with periodontitis and help clinicians make better clinical decisions.
Keywords: hypertension, National Health and Nutrition Examination Survey, NHANES), nomogram, periodontitis, prediction model
1. Introduction
Increasing degradation of the structures supporting the teeth is a hallmark of periodontitis, a chronic inflammatory condition brought on by ecologically unsound plaque biofilms.[1] Over the past 3 decades, severe periodontitis has become a major and growing global health concern. Statistics show that there are over 1 billion cases of severe periodontitis worldwide, which significantly lowers the quality of life for those who are affected.[2–4] According to research, there are several additional chronic diseases like cancer, diabetes, cardiovascular disease (CVD), and neurological disorders that are associated with periodontitis.[5–13] Therefore, determining periodontitis risk is crucial for the avoidance and management of related chronic diseases.
Around 45% of people globally suffer from hypertension, which is the most common CVD.[14] Hypertension is defined as a systolic blood pressure (SBP) ≥ 130 mm Hg and/or a diastolic blood pressure (DBP) ≥ 80 mm Hg, according to the American College of Cardiology/American Heart Association Harmonization.[15] Risk factors for hypertension include obesity, increasing age, smoking and alcohol consumption, diabetes, physical activity time, lack of sleep, and psychosomatic factors.[16–24]
Periodontitis is strongly associated with hypertension and periodontitis is one of a risk factor for hypertension, which has been reported in several studies.[25,26] A Meta-analysis confirmed that periodontitis was associated with a higher risk of hypertension, especially pronounced in severe periodontitis (OR: 1.64, 95% confidence interval [CI]: 1.23–2.19).[27] A retrospective cross-sectional study concluded that adults with periodontitis had a 20% increased risk of hypertension compared to patients without periodontitis.[28] Another cohort study also showed that patients with severe periodontitis had a higher risk of hypertension (OR: 1.47, 95% CI:1.01–2.17) than those without periodontitis.[29] The mechanism between periodontitis and hypertension is mediated by inflammation, endothelial dysfunction and oxidative stress.[30] Of course, it has also been shown that endotoxemia, which can increase the risk of cardiometabolic abnormalities and the start of CVD, can be caused by metabolic abnormalities and high levels of circulating lipopolysaccharide in individuals with periodontitis.[31]
Nomogram is a useful and convenient tool that clinicians can use to predict the probability of risk, and the effectiveness of treatment.[32,33] Compared to conventional methods of evaluation, the nomogram model generates forecasts that are more precise and straightforward.[34–36] With the increasing prevalence of periodontitis in recent years, there is a need to develop a prediction model for the risk of hypertension in patients with periodontitis. However, unfortunately, there is no prediction model to assess the risk of hypertension in patients with periodontitis. Using data from the National Health and Nutrition Examination Survey (NHANES) database from 2009 to 2014, our study aimed to explore the risk factors associated with developing hypertension in patients with periodontitis and to develop and validate a nomogram clinical prediction model based on risk factors to help clinicians make personalized clinical decisions.
2. Methods
2.1. Study design and participants
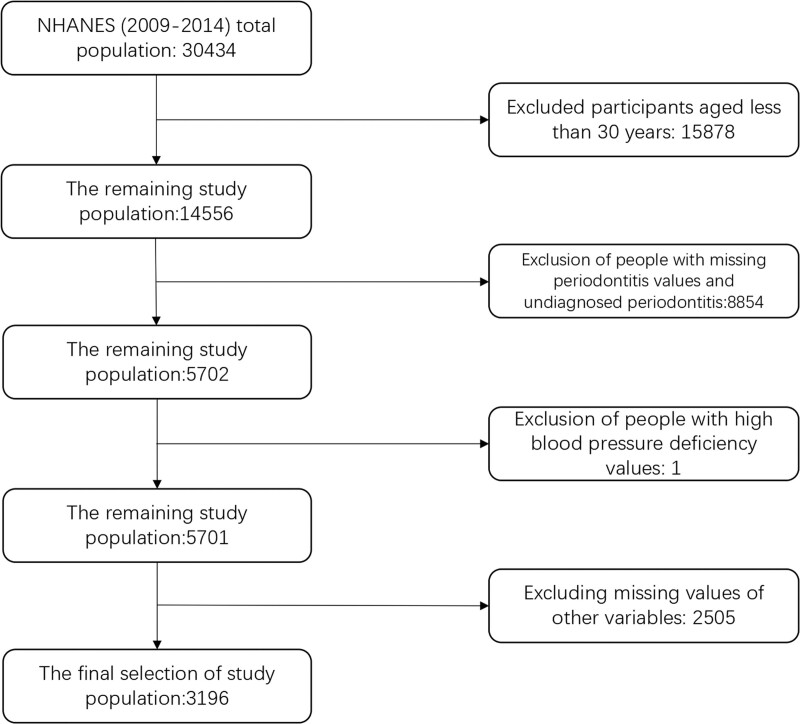
The NHANES, released every 2 years to gather a nationally representative sample of non-institutional civilians in the United States, is a multi-stage stratified probability design for a public database documenting the health and nutritional status of the U.S. population. Data from these samples included demographic data, dietary data, physical measurements, laboratory data, and questionnaire data. We extracted data from a total of 30,434 participants from 3 cycles of the NHANES (2009–2010, 2011–2012, 2013–2014). The population we selected for the study was that of subjects aged 30 years and older who had ever been diagnosed with periodontitis. Exclusion criteria were: people with undiagnosed periodontitis; age <30 years old; people with at least one missing value in all data. Ultimately, after inclusion and exclusion, 3196 participants with periodontitis constituted our study population. The flow chart of participant screening is shown in Figure 1.
Figure 1.
The Flowchart of participants.
2.2. Ethical approval
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All information from the NHANES program is available and free for public, so the agreement of the medical ethics committee board was not necessary. All the participants signed the written informed consent before participating in the study.
2.3. The definition of periodontitis
A dental hygienist evaluated the participants’ periodontal health by conducting a full-mouth periodontal examination. If a participant was 30 years of age or older, had at least one tooth (excluding the third molar), and did not satisfy any of the health exclusion criteria, they were eligible for a periodontal assessment.[37] Using the AAP/CDC criteria, periodontitis was identified and its severity was categorized as nonexistent, mild, moderate, or severe. The total number of instances of periodontitis is the sum of the cases that are mild, moderate, and severe.
2.4. The definition of hypertension
The ACC/AHA definition of hypertension is a systolic (SYS) blood pressure >130 mm Hg and/or a diastolic (DIA) blood pressure in excess of 80 mm Hg.[38] The NHANES staff asked the participant, “have you ever been told by a doctor or other health professional that you had hypertension” and if the participant answered, “yes,” the participant was considered to have hypertension.
2.5. Other variables
The data used in this study included demographics (age, gender, race, education, marital status, poverty to income ratio), anthropometric data (waist circumference [WC], body mass index [BMI], SBP, DBP), self-reported conditions (sleep time on workdays, sedentary time, physical activity time, diabetes status, and use of antihypertensive medications), and various biochemical indicators. Demographic characteristics were categorized by gender (male, female), race (non-Hispanic white, non-Hispanic black, Mexican American, Hispanic American, other race), marital status (unmarried, married or living with partner, married but currently living alone [separated, divorced, or widowed]), and education level (<9th grade, 9th-11th grade, high school graduate, partial college or AA graduate or above). The household (or individual) income was divided by the survey year and the state-specific poverty line to determine the poverty-to-income ratio. WC and BMI were obtained by medical staff at mobile screening stations. BMI was calculated by dividing weight in kilograms by the square of height in meters (kg/m2). SBP and DBP were measured at the mobile screening station on the day of the survey. The individuals were questioned, “How much time do you typically sit in a day?” to determine the amount of sedentary time. The sleep time on workdays was determined by asking participants, “How much sleep do you typically receive on a workday?.” Physical activity time was obtained by asking the subjects “how long they exercised in a week.” The smoking status of each participant was assessed by self-report, and they were divided into 3 groups: nonsmokers, former smokers, and current smokers. Drinking status was categorized according to the degree of drinking as never drinking, former drinkers now abstaining, heavy drinkers (≥3 drinks per day for women/≥4 drinks per day for men/five or more days of binge drinking per puff), moderate drinkers (≥2 drinks per day for women/≥3 drinks per day for men/≥2 days of binge drinking per puff), and light drinkers (excluding the above). Diabetes status is also known by self-report and can be divided into 3 categories: no, prediabetes, yes. There are 3 antihypertensive drug use states: not taking antihypertensive drugs, taking glucose-lowering drugs, and taking other prescribed medications but not antihypertensive drugs. Some other blood specimens such as uric acid (UA), creatinine (CR), blood urea nitrogen (BUN), albumin, triglycerides (TG), total cholesterol, high density lipoprotein, aspartate aminotransferase, alanine aminotransferase and HbA1c are measured from the laboratory.
2.6. Development and validation of nomogram
The data set is divided into a training set and a validation set in the ratio of 7:3. The training set is used to build the nomogram, and the validation set is used for validation. Because of the complex sampling characteristics of NHANES, we used NHANES sample weights in our baseline information and logistic regression analysis to obtain nationally representative estimates. Based on the results of weighted univariate logistic regression analysis with P < .05, variables with P < .05 were included for weighted multivariate logistic regression analysis. As effect estimates, the ratio (OR) and 95% CI were used. Nomogram was constructed on the training set based on the results of multivariate logistic regression analysis. On the validation set we perform validation to determine the performance of the nomogram. The subject operating characteristic curve was used to calculate the areas under the receiver operating characteristic (ROC) curve (AUC) and to evaluate the discrimination of the prediction model. Calibration curve was used to determine the relationship between the actual probability and the predicted probability. In addition, decision curve analysis (DCA) and clinical impact curve (CIC) were used to evaluate the clinical application value of nomogram.
2.7. Statistical analysis
Because the goal of NHANES is to generate data representative of the non-institutional civilian population in the United States, all estimates were calculated using sample weights in accordance with NCHS analysis guidelines. Continuous variables are expressed as means and standard errors, and categorical variables are expressed as counts and percentages. Continuous variables were compared between 2 groups using t-test and categorical variables were compared between 2 groups using chi-square test or Fisher exact test to determine the differences between groups. All statistical analysis were performed in the R software (version 4.3.0, https://www.r-project.org/). P < .05 was considered statistically significant.
3. Results
3.1. Demographic baseline characteristics
The mean age of the subjects, who made up 3196 total cases of periodontitis, was 54.15 years; there were 1199 women and 1997 men among them. Non-Hispanic whites made up 41.4% of the population, non-Hispanic blacks 22.93%, Mexican-Americans 16.15%, Hispanics 9.17%, and other races 10.36%. There were 1980 individuals with a diagnosis of hypertension and another 1216 without a diagnosis of hypertension. A total of 2238 subjects with periodontitis were included in the training set and 958 in the validation set. The training and validation sets were divided into 2 groups according to whether they were diagnosed with hypertension or not. In the training set, statistically significant differences were found between those diagnosed with hypertension and those not diagnosed with hypertension in terms of age, race, education, BMI, WC, UA, CR, BUN, TG, SYS, DIA, HbA1c, smoking status, diabetes status, and medication use (P < .05). In the validation set, there was statistical significance between the 2 groups in terms of age, BMI, WC, UA, CR, TG, SYS, DIA, HbA1c, aspartate aminotransferase, diabetes mellitus status, and use of antihypertensive drugs (P < .05). Detailed weighted baseline information on the study population is shown in Table 1.
Table 1.
Weighted baseline characteristics for participants.
| Variables | Total | Training No | Set Yes | P value | Validation No | Set Yes | P value |
|---|---|---|---|---|---|---|---|
| N | 3196 | 852 | 1386 | 364 | 594 | ||
| AGE (yr) | 54.15 (0.37) | 50.35 (0.56) | 57.01 (0.50) | <.001 | 50.19 (0.78) | 56.69 (0.56) | <.001 |
| BMI (kg/m²) | 28.79 (0.16) | 27.77 (0.25) | 29.53 (0.23) | <.001 | 27.74 (0.33) | 29.51 (0.32) | <.001 |
| WC (cm) | 100.43 (0.37) | 97.26 (0.61) | 102.77 (0.54) | <.001 | 97.37 (0.78) | 102.49 (0.87) | <.001 |
| PIR | 2.92 (0.06) | 2.97 (0.07) | 2.88 (0.08) | .25 | 2.94 (0.16) | 2.94 (0.11) | .98 |
| SLEEP (h) | 6.81 (0.03) | 6.89 (0.05) | 6.77 (0.04) | .06 | 6.73 (0.10) | 6.84 (0.07) | .38 |
| Sedentary (min) | 349.69 (5.37) | 343.63 (9.24) | 354.27 (7.19) | .34 | 354.50 (13.86) | 345.88 (13.93) | .69 |
| Exercise (min) | 647.33 (20.12) | 687.83 (41.79) | 609.16 (27.65) | .07 | 673.52 (59.25) | 647.90 (40.46) | .75 |
| UA (mg/dL) | 5.57 (0.04) | 5.30 (0.05) | 5.79 (0.06) | <.001 | 5.20 (0.11) | 5.88 (0.34) | <.001 |
| CR (mg/dL) | 0.91 (0.01) | 0.89 (0.01) | 0.94 (0.01) | <.001 | 0.88 (0.01) | 0.92 (0.01) | .01 |
| BUN (mg/dL) | 13.70 (0.14) | 13.11 (0.16) | 14.28 (0.21) | <.001 | 13.02 (0.32) | 13.87 (0.33) | .09 |
| ALB (mg/dL) | 4.27 (0.01) | 4.28 (0.01) | 4.26 (0.01) | .21 | 4.26 (0.02) | 4.27 (0.02) | .63 |
| TG (mg/dL) | 163.85 (3.38) | 145.59 (5.33) | 174.81 (5.69) | <.001 | 145.59 (7.87) | 181.63 (10.58) | .004 |
| TCHOL (mg/dL) | 199.65 (1.12) | 197.51 (2.16) | 199.05 (1.52) | .53 | 200.15 (2.71) | 203.94 (2.16) | .27 |
| HDL (mg/dL) | 53.25 (0.46) | 53.57 (0.85) | 52.27 (0.62) | .24 | 53.46 (0.99) | 54.73 (1.43) | .46 |
| Systolic (mm Hg) | 124.79 (0.49) | 113.26 (0.40) | 133.13 (0.90) | <.001 | 113.36 (0.88) | 132.85 (0.81) | <.001 |
| Diastolic (mm Hg) | 71.68 (0.35) | 67.40 (0.37) | 75.03 (0.57) | <.001 | 66.60 (0.53) | 74.74 (0.75) | <.001 |
| HbA1c (%) | 5.78 (0.03) | 5.60 (0.03) | 5.95 (0.04) | <.001 | 5.60 (0.05) | 5.81 (0.03) | <.001 |
| AST (mg/dL) | 27.04 (0.42) | 26.38 (0.96) | 27.90 (0.81) | .22 | 25.24 (0.73) | 27.50 (0.50) | .01 |
| ALT (mg/dL) | 26.81 (0.56) | 26.21 (0.98) | 27.93 (0.95) | .19 | 24.47 (1.22) | 26.97 (0.82) | .08 |
| Education | .001 | .47 | |||||
| <9th grade | 321 (10.04%) | 91 (6.59%) | 140 (5.57%) | 37 (4.77%) | 53 (4.73%) | ||
| 9–11th grade | 504 (15.77%) | 115 (10.00%) | 237 (13.36%) | 56 (14.50%) | 96 (13.09%) | ||
| High school graduate | 802 (25.09%) | 189 (22.18%) | 381 (27.96%) | 86 (21.91%) | 146 (23.39%) | ||
| Some college graduate | 888 (27.78%) | 237 (29.27%) | 372 (29.20%) | 100 (29.98%) | 179 (35.99%) | ||
| College graduate or above | 681 (21.31%) | 220 (31.96%) | 256 (23.90%) | 85 (28.85%) | 120 (22.81%) | ||
| MARITAL | .32 | .38 | |||||
| Never married | 340 (10.64%) | 99 (10.56%) | 128 (8.68%) | 52 (13.47%) | 61 (11.01%) | ||
| Living with partner | 2020 (63.2%) | 560 (66.44%) | 863 (65.19%) | 235 (65.40%) | 362 (63.36%) | ||
| Widowed/divorced | 836 (26.16%) | 193 (23.00%) | 395 (26.13%) | 77 (21.13%) | 171 (25.64%) | ||
| RACE | <.001 | .07 | |||||
| Non-Hispanic White | 1323 (41.4%) | 356 (66.11%) | 550 (66.04%) | 161 (67.83%) | 256 (68.29%) | ||
| Non-Hispanic Black | 733 (22.93%) | 136 (9.22%) | 375 (14.51%) | 64 (9.08%) | 158 (13.96%) | ||
| Mexican American | 516 (16.15%) | 176 (12.41%) | 200 (8.18%) | 65 (9.92%) | 75 (6.59%) | ||
| Other Hispanic | 293 (9.17%) | 85 (5.65%) | 135 (5.14%) | 28 (4.32%) | 45 (3.10%) | ||
| Other race | 331 (10.36%) | 99 (6.60%) | 126 (6.13%) | 46 (8.84%) | 60 (8.06%) | ||
| SEX | .11 | .35 | |||||
| Female | 1199 (37.52%) | 335 (39.41%) | 494 (35.31%) | 150 (42.92%) | 220 (39.05%) | ||
| Male | 1997 (62.48%) | 517 (60.59%) | 892 (64.69%) | 214 (57.08%) | 374 (60.95%) | ||
| SMOKE | .03 | .23 | |||||
| Never | 1507 (47.15%) | 431 (50.25%) | 644 (45.14%) | 157 (40.51%) | 275 (44.20%) | ||
| Former | 897 (28.07%) | 199 (24.27%) | 422 (31.78%) | 100 (28.34%) | 176 (31.48%) | ||
| Now | 792 (24.78%) | 222 (25.48%) | 320 (23.08%) | 107 (31.15%) | 143 (24.32%) | ||
| ALCOHOL | .76 | .77 | |||||
| Never | 380 (11.89%) | 97 (7.98%) | 177 (8.79%) | 43 (9.68%) | 63 (8.81%) | ||
| Former | 626 (19.59%) | 150 (17.35%) | 298 (18.53%) | 71 (17.45%) | 107 (15.89%) | ||
| Mild | 1109 (34.7%) | 296 (35.95%) | 465 (37.74%) | 122 (35.13%) | 226 (36.20%) | ||
| Moderate | 422 (13.2%) | 116 (16.42%) | 180 (14.49%) | 81 (20.01%) | 119 (23.94%) | ||
| Heavy | 659 (20.62%) | 193 (22.29%) | 266 (20.45%) | 47 (17.74%) | 79 (15.16%) | ||
| DM | <.001 | <.001 | |||||
| No | 2244 (70.21%) | 691 (85.48%) | 866 (67.52%) | 300 (85.24%) | 387 (69.47%) | ||
| Prediabetes | 287 (8.98%) | 48 (4.63%) | 141 (10.45%) | 25 (6.00%) | 73 (13.50%) | ||
| Yes | 665 (20.81%) | 113 (9.89%) | 379 (22.03%) | 39 (8.76%) | 134 (17.03%) | ||
| Take drug for hypertension | <.001 | <.001 | |||||
| No | 1286 (40.24%) | 483 (51.77%) | 405 (27.18%) | 216 (50.50%) | 182 (28.16%) | ||
| Yes | 257 (8.04%) | 3 (0.22%) | 188 (14.62%) | 2 (0.37%) | 64 (10.69%) | ||
| Other | 1653 (51.72%) | 366 (48.00%) | 793 (58.20%) | 146 (49.13%) | 348 (61.15%) |
ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, BUN = blood urea nitrogen, CR = creatinine, DM = diabetes mellitus, HDL = high density lipoprotein, PIR = poverty-to-income ratio, TCHOL = total cholesterol, TG = triglyceride, UA = uric acid, WC = waist circumference.
3.2. Univariate and multivariable logistic regression analysis results
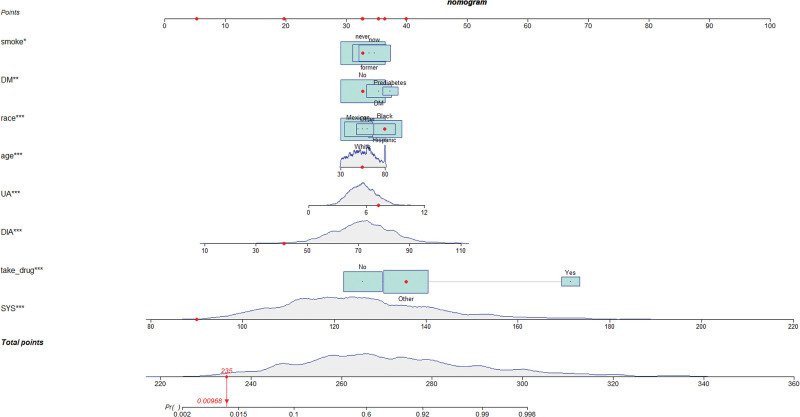
Univariate logistic analysis showed that risk factors such as age, race, marital status, BMI, WC, UA, CR, BUN, TG, HbA1c, SYS, DIA, smoking status, and antihypertensive drug use were statistically significant (P < .05). The variables that were statistically significant in the univariate logistic analysis were included in the multivariate logistic regression analysis, and the results showed that 8 variables, including age, race, UA, SYS, DIA, smoking status, diabetes status, and use of antihypertensive medication, were independent risk factors for hypertension risk in the periodontitis population (P < .05). The results of univariate logistic analysis and multivariate logistic analysis are shown in Table 2.
Table 2.
Weighted univariate and multivariate logistic regression analysis.
| Variables | Univariate OR (95% CI) | P value | Multivariate OR (95% CI) | P value |
|---|---|---|---|---|
| AGE (yr) | 1.04 (1.03,1.05) | <.001 | 1.02 (1.01, 1.04) | .004 |
| BMI (kg/m²) | 1.05 (1.04,1.07) | <.001 | / | / |
| WC (cm) | 1.03 (1.02,1.03) | <.001 | / | / |
| PIR | 0.98 (0.93,1.03) | .39 | / | / |
| SLEEP (h) | 0.98 (0.91,1.04) | .45 | / | / |
| Sedentary (min) | 1.00 (1.00,1.00) | .62 | / | / |
| Exercise (min) | 1.00 (1.00,1.00) | .07 | / | / |
| UA (mg/dL) | 1.33 (1.23,1.45) | <.001 | 1.29 (1.17, 1.42) | <.001 |
| CR (mg/dL) | 2.32 (1.46,3.69) | <.001 | ||
| ALB (mg/dL) | 0.93 (0.74,1.17) | .53 | ||
| BUN (mg/dL) | 1.04 (1.02,1.07) | <.001 | ||
| TCHOL (mg/dL) | 1.00 (1.00,1.00) | .33 | ||
| TG (mg/dL) | 1.00 (1.00,1.00) | <.001 | ||
| HDL (mg/dL) | 1.00 (0.99,1.00) | .61 | ||
| AST (mg/dL) | 1.01 (1.00,1.02) | .13 | ||
| ALT (mg/dL) | 1.01 (1.00,1.01) | .10 | ||
| HbA1c (%) | 1.50 (1.28,1.77) | <.001 | ||
| Systolic (mm Hg) | 1.14 (1.12,1.15) | <.001 | 1.13 (1.11, 1.15) | <.001 |
| Diastolic (mm Hg) | 1.07 (1.05,1.08) | <.001 | 1.07 (1.05, 1.09) | <.001 |
| Education | ||||
| <9th grade | Ref. | Ref. | Ref. | Ref. |
| 9–11th grade | 1.32 (0.90,1.94) | .15 | / | / |
| High school graduate | 1.36 (0.96,1.94) | .08 | / | / |
| Some college graduate | 1.21 (0.81,1.81) | .35 | / | / |
| College graduate or above | 0.86 (0.59,1.26) | .44 | / | / |
| MARITAL | ||||
| Never married | Ref. | Ref. | Ref. | Ref. |
| Living with Partner | 1.19 (0.86,1.64) | .28 | / | / |
| Widowed/Divorced | 1.41 (1.05,1.90) | .02 | / | / |
| RACE | ||||
| Non-Hispanic White | Ref. | Ref. | Ref. | Ref. |
| Non-Hispanic Black | 1.56 (1.25,1.95) | <.001 | 1.70 (1.14,2.52) | .01 |
| Mexican American | 0.66 (0.48,0.90) | .01 | 0.93 (0.60,1.45) | .75 |
| Other Hispanic | 0.86 (0.66,1.11) | .24 | 1.77 (1.01,3.11) | .047 |
| Other race | 0.92 (0.68,1.25) | .59 | 1.23 (0.71,2.14) | .44 |
| SEX | ||||
| Female | Ref. | Ref. | Ref. | Ref. |
| Male | 1.18 (0.98,1.43) | .07 | / | / |
| SMOKE | ||||
| Never | Ref. | Ref. | Ref. | Ref. |
| Former | 1.31 (1.04,1.64) | .02 | 1.42 (1.04,1.93) | .029 |
| Now | 0.91 (0.72,1.15) | .41 | 1.43 (0.99,2.06) | .054 |
| ALCOHOL | ||||
| Never | Ref. | Ref. | Ref. | Ref. |
| Former | 0.98 (0.68,1.42) | .93 | / | / |
| Mild | 1.01 (0.71,1.44) | .96 | / | / |
| Moderate | 0.84 (0.57,1.25) | .39 | / | / |
| Heavy | 0.97 (0.72,1.29) | .81 | / | / |
| DM | ||||
| No | Ref. | Ref. | Ref. | Ref. |
| Prediabetes | 2.83 (1.90,4.21) | <.001 | 2.51 (1.60,3.94) | <.001 |
| Yes | 2.69 (1.99,3.62) | <.001 | 1.35 (0.75, 2.43) | .298 |
| Take drug for Hypertension | ||||
| No | Ref. | Ref. | ||
| Yes | 92.71 (35.57,241.67) | <.001 | 254.85 (81.22,793.46) | <.001 |
| Other | 2.29 (1.83, 2.86) | <.001 | 3.04 (2.16,4.30) | <.001 |
ALB = albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, BUN = blood urea nitrogen, CI = confidence interval, CR = creatinine, DM = diabetes mellitus, HDL = high density lipoprotein, PIR = poverty-to-income ratio, TCHOL = total cholesterol, TG = triglyceride, UA = uric acid, WC = waist circumference.
3.3. Development and validation of nomogram for risk of hypertension in populations with periodontitis
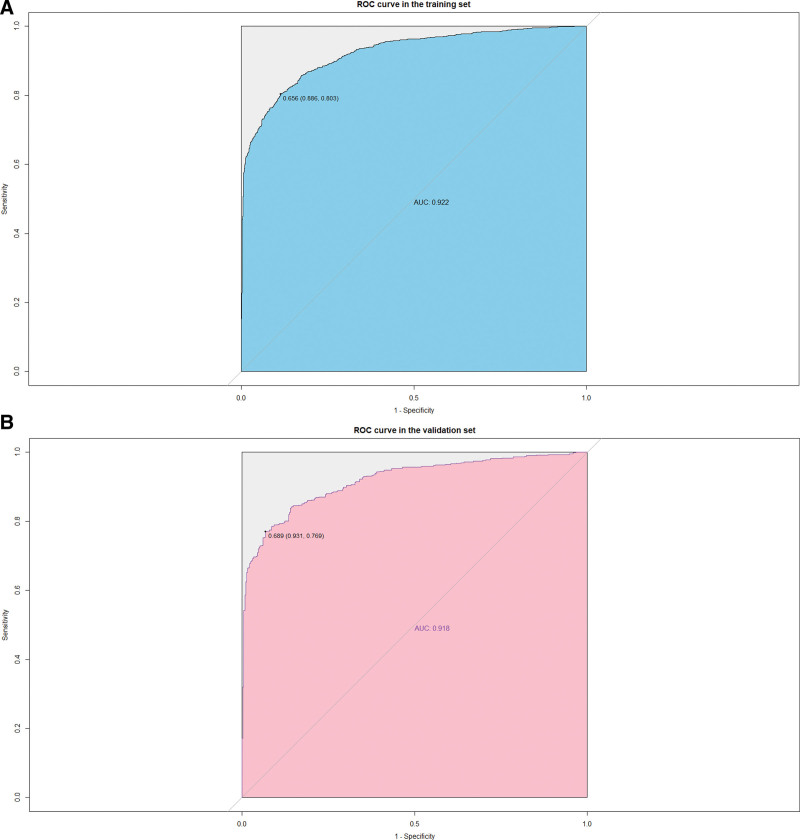
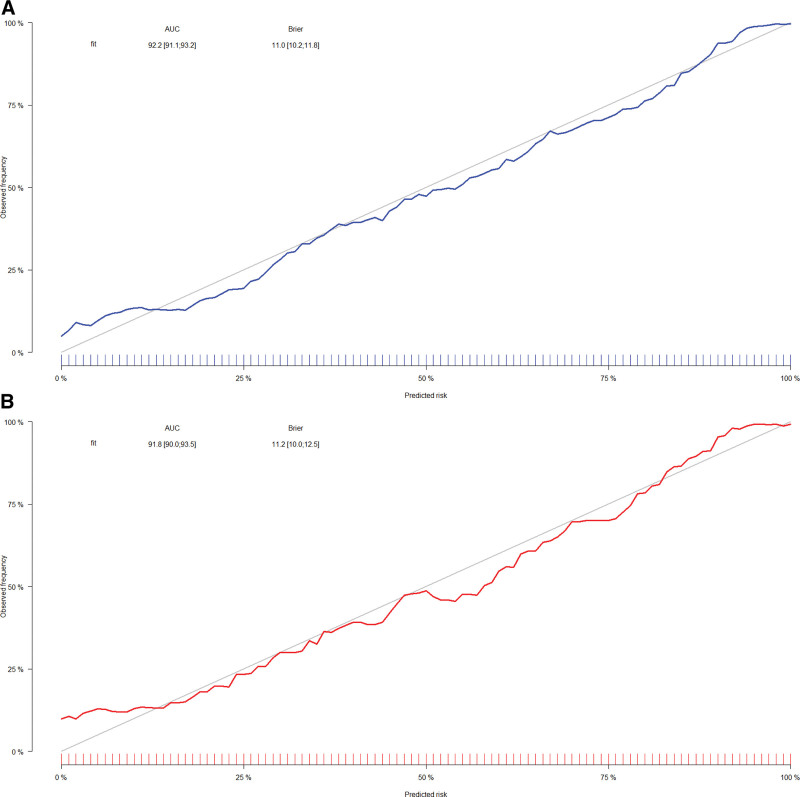
Univariate and multivariate logistic regression results (P < .05) were used to identify 8 predictors of age, race, UA, SYS, DIA, smoking status, diabetic status, and use of antihypertensive medications, on the basis of which we constructed a nomogram of hypertension risk in patients with periodontitis on the training set (Fig. 2). The ROC curve was used to evaluate the discrimination of the model. The results showed that the AUC was 0.922 (95% CI: 0.911–0.933), the sensitivity was 0.803, the specificity was 0.886 and the cutoff value was 0.656 on the training set (Fig. 3A). In the validation set, the AUC was 0.918 (95% CI: 0.900–0.935), the sensitivity was 0.769, the specificity was 0.931, and the cutoff value was 0.689 (Fig. 3B). It indicates that nomogram has an excellent predictive ability. The calibration plots of the training set and validation set are in good consistency, which indicates that the prediction results are close to the actual situation (Fig. 4A and B).
Figure 2.
Nomogram for the risk of hypertension for patients with periodontitis.
Figure 3.
(A) Receiver operating characteristic (ROC) curve for training set. (B) ROC curve for validation set.
Figure 4.
(A) Calibration curve for training set. (B) Calibration curve for validation set.
3.4. Clinical utility evaluation of the nomogram
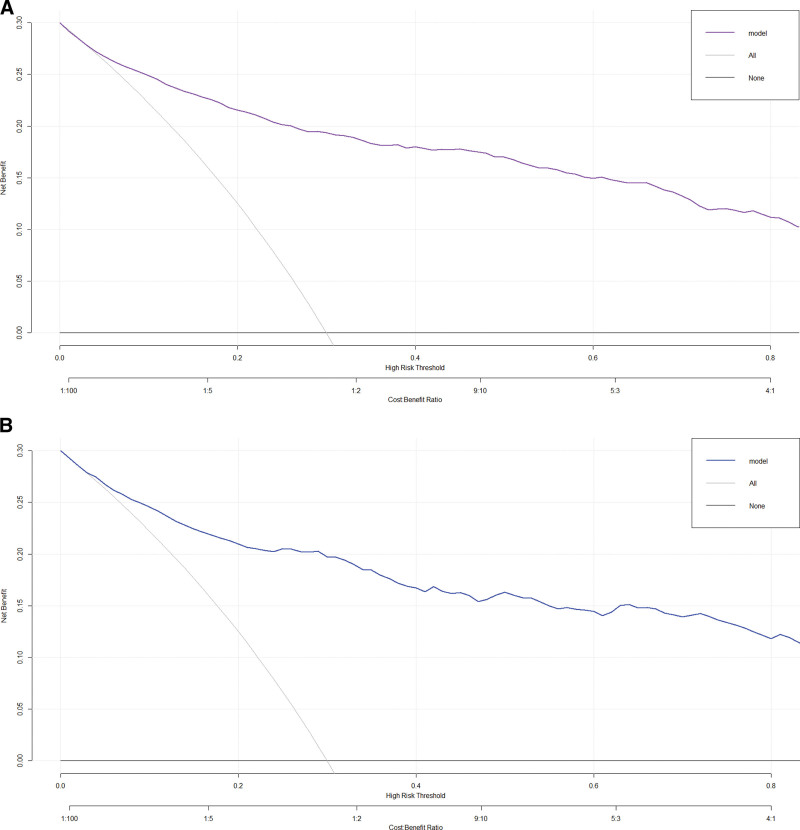
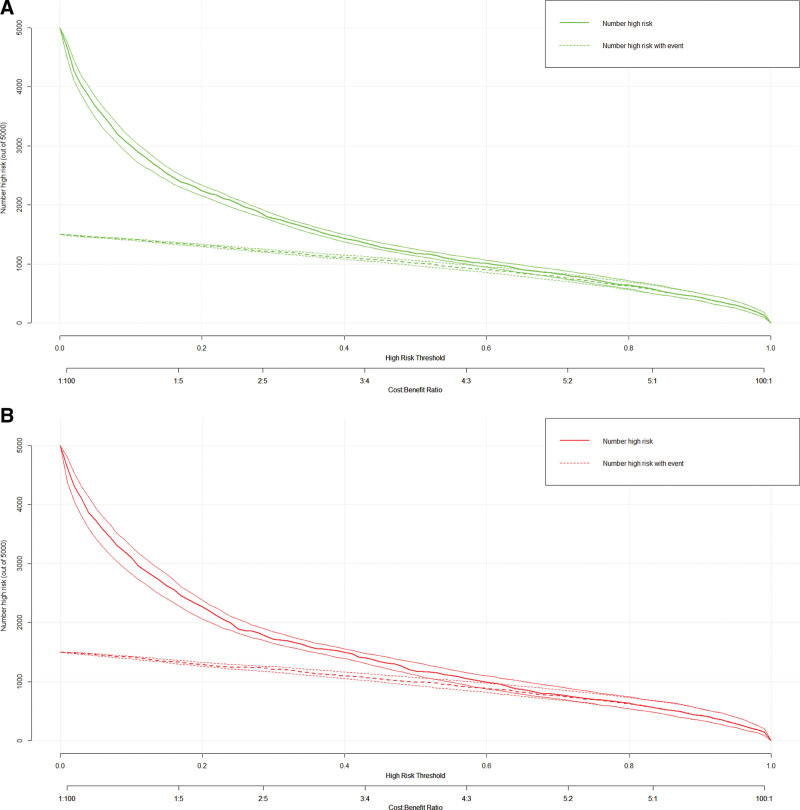
When the threshold value is higher, the net benefit of nomogram decision curve for both training set and validation set is also higher, which can indicate the better clinical utility of our nomogram (Fig. 5A and B). In addition, clinical impact plots on the training set and validation set show that there are always more expected high-risk patients than actual hypertensive patients within the most favorable threshold probability, which is accompanied by an acceptable cost-benefit ratio (Fig. 6A and B).
Figure 5.
(A) Decision curve analysis (DCA) for training set. (B) DCA for validation set.
Figure 6.
(A) Clinical impact curve (CIC) for training set. (B) CIC for validation set.
4. Discussion
For the prevention, treatment, and prognosis of hypertension risk in the periodontitis population, early warning and early management are crucial. In this study, we used data from 3196 participants with periodontitis from NHANES 2009 to 2014 to explore significant predictors of hypertension risk and to develop and validate a risk prediction model. After multivariate logistic regression analysis, 8 factors were found to be significantly associated with the risk of hypertension in patients with periodontitis, including age, race, SYS, DIA, UA, smoking status, diabetes status, and taking antihypertensive medication. A nomogram was created to predict the risk of hypertension in patients with periodontitis based on the screened variables. Additionally, the model demonstrated strong discrimination strength, calibration degree, and clinical utility, as demonstrated by ROC curve, calibration curve, DCA, and CIC.
According to our study, age was positively connected with the likelihood of developing hypertension in individuals with periodontitis. In both the training set and validation set, subjects with diagnosed hypertension had an average age that was 5 to 6 years higher than that of subjects without diagnoses. Numerous studies have revealed that age has an impact on hypertension.[39] The specific mechanism by which aging affects hypertension is that as we age, the gradual hardening of the big arteries, the gradual loss of vascular elasticity, and body system malfunctions increase vulnerability to a variety of age-dependent disorders, ultimately resulting in elevated blood pressure.[40,41] Therefore, people with periodontitis should have regular medical checkups for early prevention and treatment of the progression of hypertension. In our study, racial disparities’ impact on hypertension was also clear. Compared to white Americans, people of color and Hispanics were at a noticeably increased risk of having hypertension. Non-Hispanic black adults are more likely than non-Hispanic white adults to develop hypertension early in life and have a higher prevalence of the condition than non-Hispanic white individuals.[42] Currently, it is believed that this variation between races is caused by sympathetic overactivity, salt sensitivity, and suppression of the renin-angiotensin-aldosterone pathway.[43,44]
Many other risk factors for hypertension have also been studied in previous studies. Some studies have focused on the relationship between UA and hypertension, and 1 NHANES-based study found a positive association between UA levels and the incidence of hypertension in middle-aged and older adults.[45] And a cross-sectional study based on a Chinese population also showed that UA was dose-response associated with hypertension in the Chinese hospitalized population.[46] Our results support the findings of prior studies showing UA content is positively correlated with the risk of hypertension. There are a number of mechanisms underlying the beneficial association between UA and hypertension, including decreased endothelial nitric oxide, activation of the renin-angiotensin system, and renal microvascular disease brought on by smooth muscle cell proliferation, inflammation, and local renin-angiotensin system activation.[45,47] A considerable number of studies point to a higher risk of hypertension from smoking than from non-current smoking. Based on our study, the risk of developing hypertension was more than 40% higher for those who smoked compared to those who never smoked. Therefore, clinicians should advise subjects to improve their poor lifestyles and advocate the benefits of quitting smoking. Diabetes mellitus is a common risk factor for hypertension. Multivariate logistic regression analysis showed that diabetes and prediabetes greatly increased the risk of hypertension compared to those with normal blood glucose levels. An estimated 42.2 million persons worldwide have diabetes, which is a metabolic disease with a high global prevalence.[48] Studies have shown that diabetes decreases the compliance of large blood vessels and microvascular, reducing their ability to expand elastically, thereby affecting SYS and DBP. The synergistic effect of the coexistence of the 2 diseases leads to an increase in microvascular and macrovascular pathology and cardiovascular mortality.[49]
The benefit of nomogram is that they simplify and facilitate prediction by giving a straightforward graphical depiction of disease probabilities, in addition to visualizing the pertinent markers influencing the outcome in a multivariate regression analysis.[50] Our study is the first that we are aware of that uses the nomogram as a model to predict the risk of hypertension in patients with periodontitis. The created nomogram has strong predictive ability, and because it will be simpler to use in clinical practice, clinicians will be able to determine the best course of action for their patients.
However, our study has some limitations. First off, because the NHANES database is cross-sectional in nature, causality cannot be determined. Secondly, although internal validation was performed using the validation set after the study initially divided the complete dataset into a training set and a validation set, external validation was still needed to assess the validity of the model. Thirdly, a multicenter study involving participants from different nations and places would be required to guarantee the accuracy of the results because our study is based on research conducted in a U.S. population. Fourth, all of the data we used came from NHANES, and many of the variables were obtained through interviews, which may have had some impact on the actual results.
5. Conclusion
Summary, based on data from the NHANES database, we constructed a new nomogram model to predict the risk of developing hypertension in patients with periodontitis. Combining the ROC curve, calibration curve, DCA, and CIC, our nomogram can accurately assess the individualized risk of developing hypertension in the periodontitis population and help clinicians make better clinical decisions.
Author contributions
Formal analysis: Yicheng Wang.
Funding acquisition: Yan Zhang.
Methodology: Yicheng Wang.
Software: Yicheng Wang.
Validation: Yicheng Wang.
Writing – original draft: Yicheng Wang, Yan Zhang.
Writing – review & editing: Binghang Ni, Yuan Xiao, Yichang Lin, Yan Zhang.
Abbreviations:
- AUC
- areas under the receiver operating characteristic curve
- BMI
- body mass index
- BUN
- blood urea nitrogen
- CI
- confidence interval
- CIC
- clinical impact curve
- CR
- creatinine
- CVD
- cardiovascular disease
- DBP
- diastolic blood pressure
- DCA
- decision curve analysis
- DIA
- diastolic
- NHANES
- National Health and Nutrition Examination Survey
- ROC
- receiver operating characteristic
- SBP
- systolic blood pressure
- SYS
- systolic
- TG
- triglycerides
- UA
- uric acid
- WC
- waist circumference
The datasets generated during and/or analyzed during the current study are publicly available.
This work was supported by the Fuzhou Key Specialty Project (Grant number 20191005), and the Fuzhou “14th Five-Year Plan” Clinical Specialty Training and Cultivation Construction Project, (Grant number 20220103).
The authors have no conflicts of interest to disclose.
Study protocols for NHANES were approved by the NCHS ethnics review board (Protocol #2011–17, https://www.cdc.gov/nchs/nhanes/irba98.htm). All the participants signed the informed consent before participating in the study.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All information from the NHANES program is available and free for public, so the agreement of the medical ethics committee board was not necessary. All the participants signed the written informed consent before participating in the study.
How to cite this article: Wang Y, Ni B, Xiao Y, Lin Y, Zhang Y. A novel nomogram for predicting risk of hypertension in US adults with periodontitis: National Health and Nutrition Examination Survey (NHANES) 2009–2014. Medicine 2023;102:51(e36659)
Contributor Information
Yicheng Wang, Email: [email protected].
Binghang Ni, Email: [email protected].
Yuan Xiao, Email: [email protected].
Yichang Lin, Email: [email protected].
References
- [1].Bernabe E, Marcenes W, Hernandez CR, et al. Global, regional, and national levels and trends in burden of oral conditions from 1990 to 2017: a systematic analysis for the global burden of disease 2017 study. J Dent Res. 2020;99:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen MX, Zhong YJ, Dong QQ, et al. Global, regional, and national burden of severe periodontitis, 1990–2019: an analysis of the Global Burden of Disease Study 2019. J Clin Periodontol. 2021;48:1165–88. [DOI] [PubMed] [Google Scholar]
- [3].Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–60. [DOI] [PubMed] [Google Scholar]
- [4].Wu L, Zhang SQ, Zhao L, et al. Global, regional, and national burden of periodontitis from 1990 to 2019: results from the Global Burden of Disease Study 2019. J Periodontol. 2022;93:1445–54. [DOI] [PubMed] [Google Scholar]
- [5].Wu CZ, Yuan YH, Liu HH, et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liccardo D, Marzano F, Carraturo F, et al. Potential bidirectional relationship between periodontitis and Alzheimer’s disease. Front Physiol. 2020;11:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harding A, Singhrao SK. Periodontitis and dementia: a bidirectional relationship? J Dent Res. 2022;101:245–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Petrenya N, Hopstock LA, Holde GE, et al. Relationship between periodontitis and risk of cardiovascular disease: insights from the Tromsø Study. J Periodontol. 2022;93:1353–65. [DOI] [PubMed] [Google Scholar]
- [9].Rosário-Dos-Santos HL, Miranda SS, Gomes-Filho IS, et al. Periodontitis severity relationship with metabolic syndrome: a systematic review with meta-analysis. Oral Dis. 2022;29:2512–2520. [DOI] [PubMed] [Google Scholar]
- [10].Sun YQ, Richmond RC, Chen Y, et al. Mixed evidence for the relationship between periodontitis and Alzheimer’s disease: a bidirectional Mendelian randomization study. PLoS One. 2020;15:e0228206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Walther C, Wenzel JP, Schnabel RB, et al. Association between periodontitis and heart failure in the general population. ESC Heart Failure. 2022;9:4189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nasiri K, Amiri Moghaddam M, Etajuri EA, et al. Periodontitis and progression of gastrointestinal cancer: current knowledge and future perspective. Clin Transl Oncol. 2023;25:2801–2811. [DOI] [PubMed] [Google Scholar]
- [13].Chung PC, Chan TC. Association between periodontitis and all-cause and cancer mortality: retrospective elderly community cohort study. BMC Oral Health. 2020;20:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhan Y, Jiao J, Jing W, et al. Association between periodontitis and hypertension: cross-sectional survey from the Fourth National Oral Health Survey of China (2015–2016). BMJ Open. 2023;13:e068724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Whelton PK, Carey RM, Mancia G, et al. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension guidelines: comparisons, reflections, and recommendations. Circulation. 2022;146:868–77. [DOI] [PubMed] [Google Scholar]
- [16].El Meouchy P, Wahoud M, Allam S, et al. Hypertension related to obesity: pathogenesis, characteristics and factors for control. Int J Mol Sci. 2022;23:12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang Y, Zhang WQ, Tang WW, et al. The prevalence of obesity-related hypertension among middle-aged and older adults in China. Front Public Health. 8658;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Karayiannis CC. Hypertension in the older person: is age just a number? Intern Med J. 2022;52:1877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dikalov S, Itani H, Richmond B, et al. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am J Physiol Heart Circ Physiol. 2019;316:H639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fuchs FD, Fuchs SC. The effect of alcohol on blood pressure and hypertension. Curr Hypertens Rep. 2021;23:42. [DOI] [PubMed] [Google Scholar]
- [21].Sun D, Zhou T, Heianza Y, et al. Type 2 diabetes and hypertension. Circ Res. 2019;124:930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alpsoy S. Exercise and hypertension. Adv Exp Med Biol. 2020;1228:153–67. [DOI] [PubMed] [Google Scholar]
- [23].Han B, Chen WZ, Li YC, et al. Sleep and hypertension. Sleep Breath. 2020;24:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cai Y, Chen M, Zhai W, et al. Interaction between trouble sleeping and depression on hypertension in the NHANES 2005–2018. BMC Public Health. 2022;22:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. 2019;40:3459–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Muñoz Aguilera E, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020;116:28–39. [DOI] [PubMed] [Google Scholar]
- [27].Martin-Cabezas R, Seelam N, Petit C, et al. Association between periodontitis and arterial hypertension: a systematic review and meta-analysis. Am Heart J. 2016;180:98–112. [DOI] [PubMed] [Google Scholar]
- [28].Pietropaoli D, Del Pinto R, Ferri C, et al. Poor oral health and blood pressure control among US hypertensive adults. Hypertension. 2018;72:1365–73. [DOI] [PubMed] [Google Scholar]
- [29].Aremu JB, Pérez CM, Joshipura KJ. Longitudinal association between periodontitis and the risk of hypertension. Int J Dent. 2023;2023:2644623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tada A, Tano R, Miura H. The relationship between tooth loss and hypertension: a systematic review and meta-analysis. Sci Rep. 2022;12:13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pussinen PJ, Kopra E, Pietiäinen M, et al. Periodontitis and cardiometabolic disorders: the role of lipopolysaccharide and endotoxemia. Periodontology 2000 2022;89:19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gittleman H, Sloan AE, Barnholtz-Sloan JS. An independently validated survival nomogram for lower-grade glioma. Neuro Oncol. 2020;22:665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gittleman H, Lim D, Kattan MW, et al. An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG oncology RTOG 0525 and 0825. Neuro Oncol. 2017;19:669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oh SE, Seo SW, Choi MG, et al. Prediction of overall survival and novel classification of patients with gastric cancer using the survival recurrent network. Ann Surg Oncol. 2018;25:1153–9. [DOI] [PubMed] [Google Scholar]
- [35].Dong S, Yang H, Tang ZR, et al. Development and validation of a predictive model to evaluate the risk of bone metastasis in kidney cancer. Front Oncol. 7319;11:05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dong S, Li W, Tang ZR, et al. Development and validation of a novel predictive model and web calculator for evaluating transfusion risk after spinal fusion for spinal tuberculosis: a retrospective cohort study. BMC Musculoskelet Disord. 2021;22:825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Eke PI, Page RC, Wei L, et al. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Whelton PK, Carey RM, Mancia G, et al. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension guidelines: comparisons, reflections, and recommendations. J Am Coll Cardiol. 2022;80:1192–201. [DOI] [PubMed] [Google Scholar]
- [39].Wang C, Yuan Y, Zheng M, et al. Association of age of onset of hypertension with cardiovascular diseases and mortality. J Am Coll Cardiol. 2020;75:2921–30. [DOI] [PubMed] [Google Scholar]
- [40].McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Oliveros E, Patel H, Kyung S, et al. Hypertension in older adults: assessment, management, and challenges. Clin Cardiol. 2020;43:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Romero CX, Romero TE, Shlay JC, et al. Changing trends in the prevalence and disparities of obesity and other cardiovascular disease risk factors in three racial/ethnic groups of USA adults. Adv Prev Med. 2012;2012:172423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Deere BP, Ferdinand KC. Hypertension and race/ethnicity. Curr Opin Cardiol. 2020;35:342–50. [DOI] [PubMed] [Google Scholar]
- [44].Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu X, Huang J, Wu S, et al. The association between the serum uric acid level and hypertension in middle-aged and elderly adults. Cardiovasc Ther. 2021;2021:4626062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].He Y, Chen D, Xu JP, et al. Association between serum uric acid and hypertension in a large cross-section study in a Chinese population. J Cardiovasc Dev Dis. 2022;9:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Miyabayashi I, Mori S, Satoh A, et al. Uric acid and prevalence of hypertension in a general population of Japanese: ISSA-CKD Study. J Clin Med Res. 2020;12:431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Aliño-Dies M, Sánchez-Ortí JV, Correa-Ghisays P, et al. Grip strength, neurocognition, and social functioning in people with type-2 diabetes mellitus, major depressive disorder, bipolar disorder, and schizophrenia. Front Psychol. 2020;11:525231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yildiz M, Esenboğa K, Oktay AA. Hypertension and diabetes mellitus: highlights of a complex relationship. Curr Opin Cardiol. 2020;35:397–404. [DOI] [PubMed] [Google Scholar]
- [50].Lv J, Liu YY, Jia YT, et al. A nomogram model for predicting prognosis of obstructive colorectal cancer. World J Surg Oncol. 2021;19:337. [DOI] [PMC free article] [PubMed] [Google Scholar]