Name: ________________________
Haloalkanes
Written paper practice Class: ________________________
Date: ________________________
Time: 75 minutes
Marks: 63 marks
Comments:
Page 1 of 20
Q1.
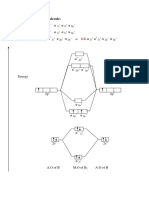
Acrylonitrile, H2C=CHCN, can be used as a starting material for the synthesis of
butane-1,4-diamine, as shown in this reaction scheme.
(a) Use IUPAC rules to name isomer W.
___________________________________________________________________
(1)
(b) Reaction 1 produces a mixture of W and two other isomers.
Draw the structures of the two other isomers.
Explain, by considering the mechanism of this reaction, why all three isomers are
formed.
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
Page 2 of 20
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(6)
The reaction scheme is repeated here.
(c) Identify the reagent that is warmed with isomer W in reaction 2.
State the other reaction condition needed.
Reagent ___________________________________________________________
Condition __________________________________________________________
Page 3 of 20
(2)
(d) State the reagent and reaction conditions needed for reaction 3.
Give an equation for reaction 3.
Reagent and conditions _______________________________________________
Equation
___________________________________________________________________
(2)
(e) An incomplete equation for the formation of nylon 4,6 from five molecules of
butane-1,4-diamine and five molecules of hexanedioic acid is shown.
Deduce the values of x and y in this equation.
x ____________________ y ____________________
(2)
(f) The figure below shows a section of the nylon 4,6 polymer molecule.
Draw, on the figure above, another section of nylon 4,6 polymer showing two
hydrogen bonds between the two sections.
Draw, on the figure above, another section of nylon 4,6 polymer showing two
hydrogen bonds between the two sections.
Page 4 of 20
(2)
(Total 15 marks)
Q2.
Ethene is an important starting point for the manufacture of plastics and pharmaceutical
chemicals. Most of the ethene used by industry is produced by the thermal cracking of
ethane obtained from North Sea gas (Reaction 1). It is also possible to make ethene
either from chloroethane (Reaction 2) or from ethanol (Reaction 3).
(a) Give essential conditions and reagents for each of Reactions 2 and 3.
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(4)
(b) Name and outline a mechanism for Reaction 2. Suggest a reason why
chloroethane is not chosen by industry as a starting material to make ethene
commercially.
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
Page 5 of 20
___________________________________________________________________
___________________________________________________________________
(5)
(c) Name and outline a mechanism for Reaction 3. Suggest why this route to ethene
may become used more commonly in the future as supplies of North Sea gas begin
to run out.
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(6)
(Total 15 marks)
Q3.
Under suitable conditions, 2-bromobutane reacts with sodium hydroxide to produce a
mixture of five products, A, B, C, D and E.
Products A, B and C are alkenes.
A is a structural isomer of B and C.
A does not exhibit stereoisomerism.
B and C are a pair of stereoisomers.
Products D and E are alcohols.
D and E are a pair of enantiomers.
(a) Give the names of the two concurrent mechanisms responsible for the formation of
the alkenes and the alcohols.
Mechanism to form alkenes ____________________________________________
Mechanism to form alcohols ___________________________________________
Page 6 of 20
(2)
(b) Define the term stereoisomers.
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(2)
(c) Deduce the name of isomer A.
Explain why A does not exhibit stereoisomerism.
Name _____________________________________________________________
Explanation ________________________________________________________
___________________________________________________________________
___________________________________________________________________
(2)
(d) Outline the mechanism for the reaction of 2-bromobutane with sodium hydroxide to
form alkene A.
(3)
(e) Deduce the name of isomer B and the name of isomer C.
Explain the origin of the stereoisomerism in B and C.
Name _____________________________________________________________
___________________________________________________________________
Explanation ________________________________________________________
___________________________________________________________________
___________________________________________________________________
(2)
(f) Draw 3D representations of enantiomers D and E to show how their structures are
related.
Page 7 of 20
(2)
(g) A student compares the rates of hydrolysis of 1-chlorobutane, 1-bromobutane and
1-iodobutane.
The suggested method is:
• add equal volumes of the three halogenoalkanes to separate test tubes
• add equal volumes of aqueous silver nitrate to each test tube
• record the time taken for a precipitate to appear in each test tube.
State and explain the order in which precipitates appear.
Order in which precipitates appear ______________________________________
___________________________________________________________________
___________________________________________________________________
Explanation ________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(2)
(Total 15 marks)
Q4.
CFCs were used as refrigerants and in aerosols.
The scientists Rowland and Molina published research in 1974 to show that CFCs are
responsible for the destruction of ozone molecules in the upper atmosphere.
A few years later, other scientists discovered that the concentration of ozone in the upper
atmosphere was decreasing.
In 1987 there was an agreement by many countries to restrict the use of CFCs.
(a) The molecule CFC-11 was commonly used as a refrigerant.
Use IUPAC rules to name CFC-11
___________________________________________________________________
(1)
(b) A molecule of CFC-11 breaks down in the upper atmosphere to form a chlorine free
Page 8 of 20
radical.
Give the equation for this reaction.
___________________________________________________________________
(1)
(c) A typical refrigerator contained 0.50 kg of CFC-11 (Mr = 137.5).
One molecule of CFC-11 causes the destruction of approximately 100 000
molecules of ozone.
Use these data to estimate the number of molecules of ozone that can be destroyed
by 0.50 kg of CFC-11
Give your answer in standard form.
The Avogadro constant, L = 6.022 × 1023 mol–1
Number of molecules of ozone ____________________
(2)
(d) State the benefit to life on Earth of ozone in the upper atmosphere.
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(1)
(e) Suggest one reason why the use of CFCs was not restricted until several years after
Rowland and Molina published their research.
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(1)
(f) CFC-11 is a greenhouse gas that can contribute to global warming.
State and explain how CFC-11 is able to contribute to global warming.
___________________________________________________________________
Page 9 of 20
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(2)
(Total 8 marks)
Q5.
This question is about the reactions of alkanes.
(a) Alkanes can be used as fuels.
Give an equation for the combustion of heptane (C7H16) in an excess of oxygen.
___________________________________________________________________
(1)
(b) Heptane can be obtained from the catalytic cracking of hexadecane (C16H34) at a
high temperature.
Identify a suitable catalyst for this process.
Give one condition other than high temperature.
Give an equation for the catalytic cracking of one molecule of hexadecane to
produce one molecule of heptane, one molecule of cyclohexane and one other
product.
Catalyst ____________________________________________________________
Condition ___________________________________________________________
Equation ___________________________________________________________
___________________________________________________________________
(3)
(c) Alkanes can be used in free-radical substitution reactions to produce
halogenoalkanes.
Give equations for the propagation steps in the reaction of butane to form
2-chlorobutane.
___________________________________________________________________
___________________________________________________________________
(2)
(d) Chlorofluorocarbons (CFCs) are a group of halogenoalkanes currently banned in
many countries. They cannot be used as solvents or refrigerants because of their
effect on the environment.
Page 10 of 20
The structure of a CFC is shown.
Identify the radical produced from this CFC that is responsible for the depletion of
ozone in the atmosphere.
Explain, with the aid of equations, why a single radical can cause the decomposition
of many molecules of ozone.
Radical __________________________________________________________
Explanation ________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
___________________________________________________________________
(4)
(Total 10 marks)
Page 11 of 20
Mark schemes
Q1.
(a) 3-bromopropanenitrile
Allow 3-bromopropane-1-nitrile
1
(b) This question is marked using levels of response. Refer to the
Mark Scheme Instructions for Examiners for guidance on how to
mark this question.
All stages are covered and each stage is generally
Level 3
correct and virtually complete.
5-6
Answer is communicated coherently and shows a
marks
logical progression from Stage 1 to Stages 2 and 3.
All stages are covered but stage(s) may be incomplete
or may contain inaccuracies
Level 2
OR two stages are covered and are generally correct
3-4 and virtually complete.
marks
Answer is communicated mainly coherently and shows
a logical progression from Stage 1 to Stages 2 and 3.
Two stages are covered but stage(s) may be
incomplete or may contain inaccuracies OR only one
Level 1
stage is covered but is generally correct and virtually
1-2 complete.
marks
Answer includes isolated statements but these are not
presented in a logical order.
Level 0
0 Insufficient correct chemistry to gain a mark.
marks
Indicative Chemistry content
Stage 1 Types of Isomers formed
1a CH3CHBrCN
1b Exists as two Optical isomers / enantiomers
Stage 2 Mechanism
2a 2 curly arrows
2b Intermediate structure primary carbocation OR
Page 12 of 20
2c Alternative Intermediate structure secondary carbocation OR
Stage 3 Optical isomerism
3a 2-bromo isomer has chiral carbon / C with four different
groups / non superimposable mirror images
OR
3b Optical because (secondary) C+ planar
3c So can be attacked from above or below
(c) M1 KCN or NaCN
Penalise acid in M1
M2 Aqueous AND ethanol (alcohol)
2
(d) M1 H2 and Ni/Pt/Pd
Allow LiAlH4 and (Dry) ether BUT not NaBH4
M2 NCCH2CH2CN + 4H2 → H2N(CH2)4NH2
Allow with 8[H]
2
(e) M1 x = 5
M2 y = 9
2
Page 13 of 20
(f)
Structure shown on the left of the given structure.
The correct answer is the same irrespective of whether it's
drawn on the left or right of the polymer section.
Deduct a mark(s) for error(s)/omission(s)
Must have the following:
• Minimum correct structure
Or
• Lp on O or N
• 2 Linear dashed lines from O or N to H
Allow alternative connection below
Page 14 of 20
2
[15]
Q2.
(a) Reaction 2: NaOH OR KOH (1) M1 alcohol (ic) OR ethanol (ic)(1) M2
ignore heat
Condition mark linked to correct reagent but award M2 if OH–
or base or alkali mentioned
Reaction 3: concentrated H2SO4 OR H3PO4 M1 (1) heat (1) M2
OR 150°C - 200°C
Condition mark linked to correct reagent but award M2 if
H2SO4 or H3PO4, but not concentrated
Penalise reagent and condition if dilute H2SO4 / H3PO4
4
(b) Mechanism:
Award M3 independently
M1 and M2 must be to / from correct places
E1 mechanism possible in which M2
Name: of mechanism = elimination (1)
NOT dehydrohalogenation
Ignore “base” OR “nucleophilic” before elimination
Page 15 of 20
Reason: Reaction 2 has (very) low yield (1)
5
QoL OR chloroethane has to be made (from ethane)
OR chloroethane is expensive
OR chloroethane is not redily available
(c) Mechanism:
Name of mechanism = elimination (1)
NOT dehydration alone
Reason: Ethanol could come from (fermentation of) renewable
QoL sugars / glucose / carbohydrates / sources (1)
6
[15]
Q3.
(a) (for alkenes) elimination
Allow base elimination
Not nucleophilic elimination
1
(for alcohols) nucleophilic substitution
1
(b) (Different molecules/compounds with the) same (molecular and)
structural formula
1
Different spatial arrangement of atoms
Allow different spatial arrangement of bonds/groups
1
(c) A = but-1-ene
Not butene
1
two groups/atoms/Hs the same on one of the C=C carbons
Allow two groups/atoms/Hs the same on first C
Not two groups the same on one side of C=C
Page 16 of 20
Ignore references to no chiral carbon
Ignore ‘priority’ i.e. 2 groups with the same priority… gets
M2 for ‘2 groups the same…’
1
(d)
If wrong halogenoalkane used then max 2/3
M1 lone pair on O, negative charge (anywhere) and curly
arrow from lone pair to H on carbon 1
Not if (covalent) NaOH / additional arrows to or from NaOH /
additional arrows to or from Na+
1
M2 curly arrow from C(1)−H to C(1)−C(2)
M2 is standalone from M1
Allow ecf if H on carbon 3 attacked in M1 for curly arrow
from C(3)−H to C(2)−C(3)
Not as ecf if H on carbon 2 attacked in M1 for curly arrow
from C(2)−H
1
M3 Curly arrow from C−Br to Br (mark is independent)
Not if any additional arrows / incorrect polarity or formal
charges on C−Br
1
Allow ecf for mechanism to form but-2-ene from (c)
Allow E1 mechanism
M1 curly arrow from C−Br bond to the Br
M2 curly arrow from lone pair on O of OH− to a correct H on
the correct C adjacent to C+ on the carbocation
M3 curly arrow from a correct C−H bond to a correct C−C
bond
penalise M1 for any additional arrow(s) to/from the Br to/from
anything else
penalise M2 for any additional arrow(s) on NaOH
(e) Z-but-2-ene AND E-but-2-ene
Allow ‘cis’/‘trans’ and B and C either way round
Allow E/Z but-2-ene, cis/trans but-2-ene
1
lack of/restricted/no (free) rotation around C=C/double bond
Allow C=C/double bond cannot rotate
1
(f)
Page 17 of 20
M1 any correct 2D or 3D structure of butan-2-ol
Allow C2H5
1
M2 must show at least one wedge bond and one dash bond
in each structure from the chiral C and any bonds in the
plane cannot be at 180° to each other
1
second structure could be drawn as mirror image of first or
with same orientation of bonds and two groups swapped
round
Allow ECF for second structure from incorrect first structure,
providing molecule is chiral
(g) Silver iodide then silver bromide then silver chloride
Allow yellow then cream then white
Allow iodide/AgI then bromide/AgBr then chloride/AgCl
Allow iodo(butane) then bromo(butane) then chloro(butane)
Ignore iodine then bromine then chlorine
1
bond strength C−I < C−Br < C−Cl
Ignore incorrect formulae
Allow carbon-halogen bond strength decreases down the
group / from Cl to I
1
[15]
Q4.
(a) trichlorofluoromethane
1
(b)
CCl3F → •CCl2F + •Cl
radical dot anywhere on each radical
1
(c) M1 amount of CFC-11 = ( = 3.64) mol
Allow ECF from M1 to M2
1
M2 molecules of O3 = 3.64 x 100,000 x 6.022 x 1023 = 2.19 x 1029
Allow answers in range 2 x 1029 to 2.20 x 1029 (1sf is
acceptable as this is an estimate)
1
Page 18 of 20
(d) Absorbs (harmful) ultraviolet / uv (light / radiation)
Protects us from (harmful) uv
Ignore other wavelengths / types of light
1
(e) One of these reasons:
• lack of evidence that ozone was being depleted
• lack of alternatives to CFCs
• commercial interest to continue to use CFCs
• hard to obtain international agreement
1
(f) M1 absorbs infrared radiation
M1 idea of IR being taken in
1
M2 molecule has polar bonds
M2 accept polar molecule
1
[8]
Q5.
(a) C7H16 + 11O2 → 7CO2 + 8H2O
Ignore state symbols
Allow multiples
M1
(b) Zeolite OR aluminosilicate
Allow porous pot / aluminium oxide / alumina / silica / silicon
dioxide
M1
Slight/moderate pressure
Slightly above atmospheric – allow 1-5 atmospheres /
100-500kPa
M2
C16H34 → C7H16 + C6H12 + C3H6
M3
(c)
If incorrect radical or ambiguous radical lose M1 but can
award M2 for ecf in each equation.
M1
Allow equations in either order
Allow dot anywhere on the second carbon
Ignore extra initiation and termination steps
M2
(d) Cl●
Allow Cl or Chlorine in M1 and M4
Page 19 of 20
M1
Cl● + O3 → ClO● + O2
Penalise absence of dot once in the equations
M2
ClO● + O3 → Cl● + 2O2
Allow dot anywhere on the radical
Apply the list principle in the equations and penalise
M3
Cl● is regenerated (and causes a chain reaction in the decomposition of ozone)
initiation from Cl2
Allow equations in either order.
Ignore Cl● acts as a catalyst
M4
[10]
Page 20 of 20